Background: Multiple myeloma (MM) is the 2nd most common hematologic malignancy and remains incurable despite significant treatment advances over the last decade. Patients with relapsed/refractory multiple myeloma (RRMM), who have exhausted available therapies, have limited treatment options and a median survival as brief as 6 months (Richardson PG et al., Oncology 2010). Immune checkpoint inhibitors (CPI) have dramatically changed treatment paradigms in multiple cancers, with growing evidence that radiation therapy (XRT) may synergize with these agents via the abscopal effect. MM cells express high levels of PD-L1. Preclinical models have demonstrated rejection of murine myeloma when PD-L1 blockade was combined with XRT (Kearl TJ et al., Journal of Immunology 2013), as well as longer survival in myeloma-bearing mice compared to controls (Jing W et al., Journal for ImmunoTherapy of Cancer 2015). Early phase single arm clinical trials with combinations of immunomodulatory drugs (IMiDs) and CPI showed response rates between 33 and 76% (Pianko MJ et al., Stem Cell Investigation 2017)(San Miguel J et al., Blood 2015)(Badros A et al., Blood 2017). However, subsequent phase 3 studies revealed a potential safety signal of this combination (FDA 2017). Nonetheless, a subset of patients appear to attain durable responses (Badros A et al., Blood Advances 2019). CPI combined with other therapies such as XRT, that help to prime the immune system, hold great promise in the treatment of patients with RRMM. Herein, we describe our phase II study of avelumab, an anti-PD-L1 IgG1 antibody with potential antibody-dependent cellular cytotoxic properties, in combination with XRT in patients with RRMM.
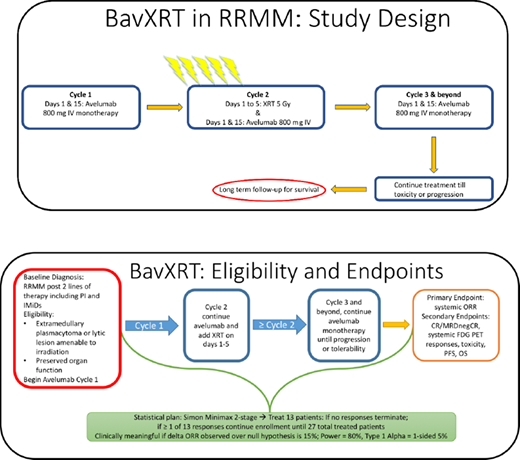
Methods: Trial Design: The primary endpoint of this trial is to assess the systemic response rate with the combination of avelumab and XRT in the treatment of extramedullary plasmacytomas or active lytic lesions in patients with RRMM using the 2016 IMWG response criteria. Secondary endpoints include determination of complete response rate, progression-free survival, and overall survival. Patients will undergo bone marrow biopsies and imaging (PET/CT and DW-MRI) at baseline, during disease response evaluations, and at the end of treatment.
Treatment: Treatment consists of a 4-week lead-in with avelumab at a flat dose of 800mg IV every 2 weeks followed by concurrent XRT of 5Gy for 5 consecutive days directed toward the plasmacytoma/lytic lesion (Figure 1). Monotherapy avelumab, 800mg IV every 2 weeks, will continue indefinitely until disease progression or unacceptable toxicity.
Analysis: This is a single arm trial with a Simon minimax two-stage phase II trial design that will enroll up to 27 patients. The first stage will enroll 13 evaluable patients, and if 0 of the 13 have a clinical response, then no further patients will be accrued due to futility. If 1 or more of the first 13 patients have a response, then accrual will continue until a total of 27 evaluable patients have been treated in the second stage. This will provide a two-sided alpha of 5% and a Power of 80% to rule out an ORR of 5% in favor of a response rate of 20%. Response fractions and time to event endpoints will be reported along with 90 and 95% two-sided confidence intervals with nominal p values.
Eligibility: Patients must have previously treated relapsed MM or RRMM refractory to, ineligible for, or intolerant of, available myeloma therapies and have ≥ 1 extramedullary plasmacytoma and/or lytic lesion. Lesions must be amenable to, and clinically indicated for, treatment with localized XRT. Eligible patients must have documented evidence of progressive disease on, or after, their most recent regimen as defined by the IMWG criteria. They must have achieved at least a minimal response to one or more prior regimens. Exclusionary criteria include patients with: clinically unstable lesions where a delay in XRT may be detrimental; active autoimmune diseases or history of serious autoimmune-related disorders; uncontrolled intercurrent illnesses; concurrent use of immunosuppressant medications; and recent or current anti-cancer treatment prior to the first dose of avelumab.
Current Enrollment: This study is actively enrolling patients to the first stage. At the time of this submission, 4 patients have been enrolled and have received at least one dose of trial therapy.
Clinical trial registry number: NCT03910439.
No relevant conflicts of interest to declare.
avelumab not approved in myeloma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal